Menu
02191008797
Urea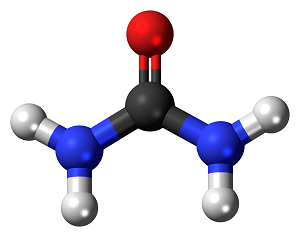 Ammonia
Ammonia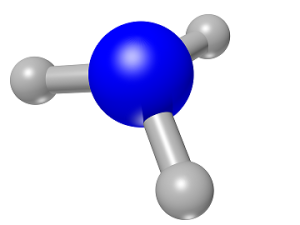
The Mission:
“Creating Value Chain from Methane Gas, Preventing National Capital Markets in Selling raw resources and Creating More Value Added investments and Moving towards the Resistive Economy”.
Enter your email to receive the latest news